11
A rigid container has volume of ![]() , and holds steam at
, and holds steam at ![]() C. 1/4 of the volume is in liquid point and the remaining at vapor form. Determine the pressure of the steam, and quality of the saturated mixture, and density of the mixture.
C. 1/4 of the volume is in liquid point and the remaining at vapor form. Determine the pressure of the steam, and quality of the saturated mixture, and density of the mixture.
Given:
Volume (V)![]()
Temperature (T)![]() C
C
Find:
- The pressure of the steam.
- The quality of the saturated mixture.
- The density of the mixture.
Solution
- From Table A-4, the properties of Temperature(T) at
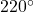 C
C 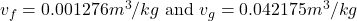
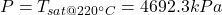
- The total mass and the quality are determined as
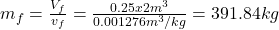
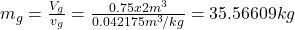
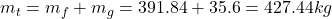
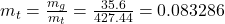
- The density is determined from
![]()
![]()
